About GARDASIL 9
About Gardasil 9
From adolescence to adulthood Gardasil 9 can help protect eligible patients from certain cancers and diseases caused by HPV later in life.3

GARDASIL® 9 helps to protect against 9 strains of human papillomavirus (HPV) disease.1
GARDASIL® 9 is indicated for active immunisation of individuals from the age of 9 years, against the following HPV diseases:1
- Premalignant lesions and cancers affecting the cervix, vulva, vagina and anus caused by vaccines HPV types
- Genital warts (Condyloma acuminata) caused by specific HPV types
What HPV types does GARDASIL® 9 help protect against?1,2
GARDASIL® 9 combines
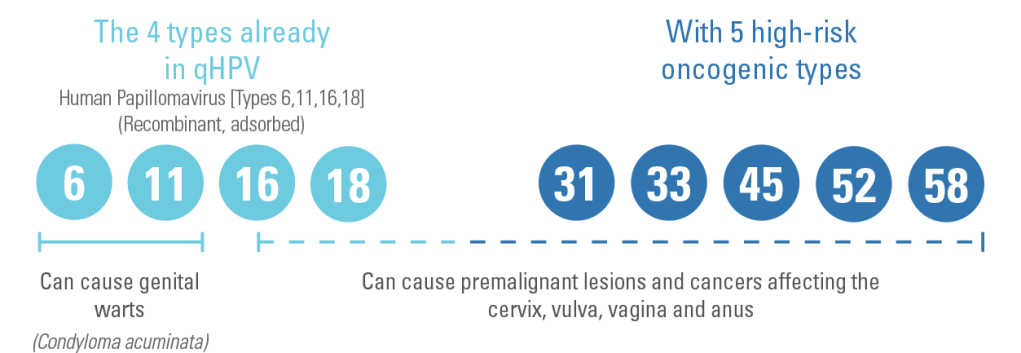
GARDASIL® 9 provides

*Phase 3 randomised, double-blind, efficacy, immunogenicity, and safety study. Participants (women aged 16 – 26 [N=14,204]) received three intramuscular injections of the GARDASIL® 9 vaccine (9vHPV) or qHPV at Day 1, Month 2, and Month 6. Efficacy was assessed after a follow-up of up to 67 months after Dose 3 (median follow-up 43 months after Dose 3). The primary outcomes were efficacy of 9vHPV vaccine versus qHPV vaccine to prevent the combined endpoint of high-grade cervical disease (cervical intraepithelial neoplasia Grade 2 or 3, adenocarcinoma in situ, invasive cervical carcinoma), vulvar disease (vulvar intraepithelial neoplasia Grade 2/3, vulvar cancer), and vaginal disease (vaginal intraepithelial neoplasia Grade 2/3, vaginal cancer) related to HPV 31, 33, 45, 52, and 58 and non-inferiority (excluding a decrease of 1·5 times) of HPV 6, 11, 16, and 18 antibody geometric mean titres (GMTs) compared with qHPV vaccine. The PPE population consisted of individuals who received all 3 vaccinations within one year of enrolment, did not have major deviations from the study protocol were naïve (PCR negative and seronegative) to the relevant HPV type(s) (31, 33, 45, 52 and 58) prior to dose one and remained PCR negative to the relevant HPV type(s) through one month post Dose 3 (month 7).
Event rate = 1/6016 in the GARDASIL® 9 group (n=7099) vs. 38/6017 in the qHPV group (n=7105)
‘Worse’ is defined as:
HPV 31-, 33-, 45-, 52-, 58-related CIN 2/3, AIS, cervical cancer, VIN 2/3, VaIN 2/3, vulvar cancer, and vaginal cancer.†
† No cases of cervical cancer, VIN2/3, vulvar and vaginal cancer were diagnosed in the PPE (Per Protocol Efficacy) population.
Please refer to the Summary of Product Characteristics for full prescribing information.
Please refer to the Summary of Product Characteristics for full prescribing information.
Hypersensitivity to the active substances or to any of the excipients. Individuals with hypersensitivity after previous administration of GARDASIL® 9 or qHPV/Silgard* should not receive GARDASIL® 9 *Silgard (Human papillomavirus vaccine [types 6, 11, 16, 18] (recombinant, absorbed)) (qHPV and Silgard is not licensed in the Republic of Ireland).
Please refer to the Summary of Product Characteristics for full prescribing information.
Safety and immunogenicity in individuals who have received immunoglobulin or blood-derived products during the 3 months prior to vaccination have not been studied in clinical trials.
Use with other vaccines
GARDASIL® 9 may be administered concomitantly with a combined booster vaccine containing diphtheria (d) and tetanus (T) with either pertussis [acellular, component] (ap) and/or poliomyelitis [inactivated] (IPV) (dTap, dT-IPV, dTap-IPV vaccines) with no significant interference with antibody response to any of the components of either vaccine. This is based on the results from a clinical trial in which a combined dTap-IPV vaccine was administered concomitantly with the first dose of GARDASIL® 9.
Use with hormonal contraceptives
In clinical studies, 60.2% of women aged 16 through 26 years who received GARDASIL® 9 used hormonal contraceptives during the vaccination period of the clinical studies. Use of hormonal contraceptives did not appear to affect the type specific immune responses to GARDASIL® 9.
Please refer to the Summary of Product Characteristics for full prescribing information.
| Side effects of HPV vaccination | |
|---|---|
| Very common side effects (occurring in ≥ 10% of people) | ● Pain, redness and swelling at the injection site ● Headache |
| Common side effects (occurring in ≥ 1% to < 10% of people) | ● Bruising, or itching at the injection site ● Dizziness ● Nausea ● Fever ● Fatigue |
A full list of side effects can be found in the Summary of Product Characteristics and Patient Information Leaflet.
Long-term effectiveness and immunogenicity (up to 14 years) with GARDASIL® and GARDASIL® 9 in older adolescent and adult females1-3
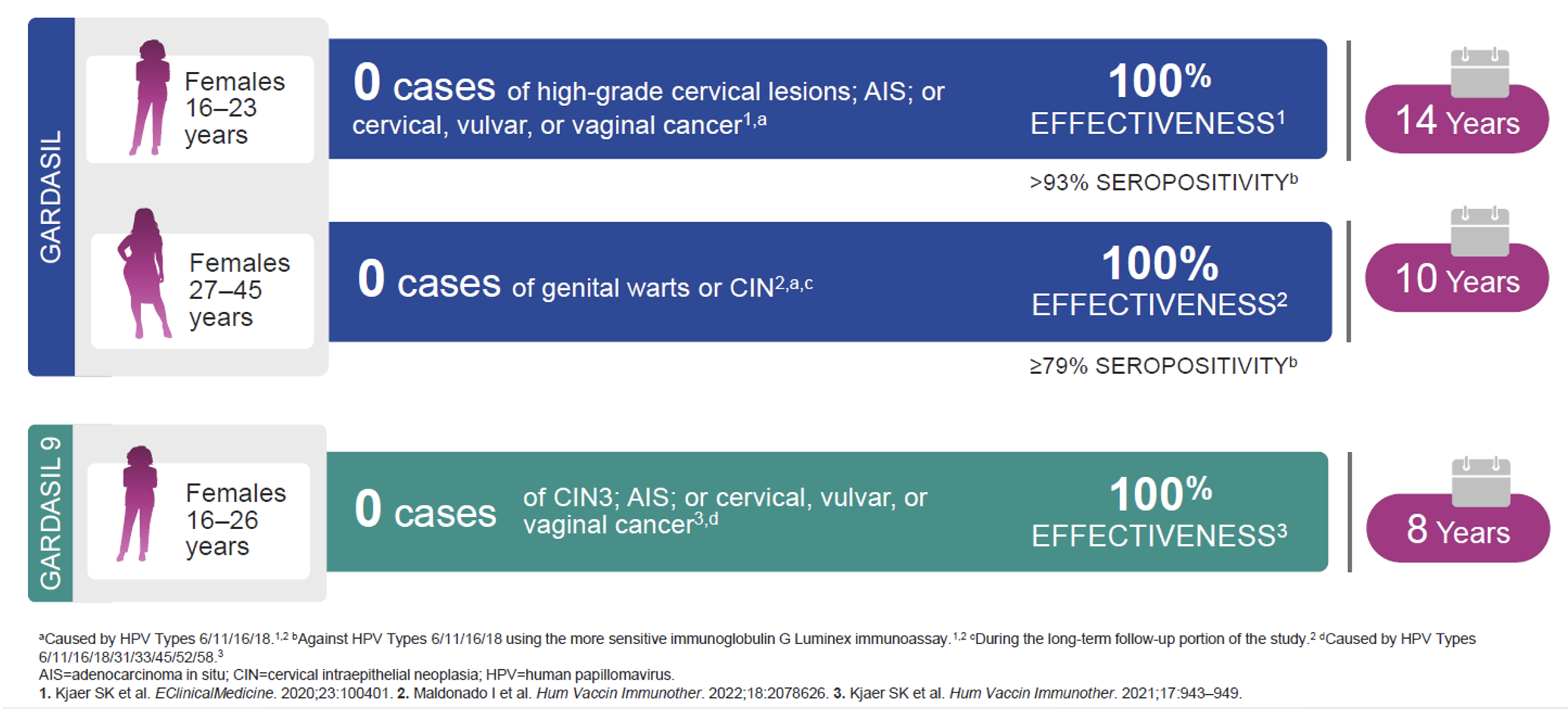
Long-term effectiveness studies
A subset of subjects is being followed up for 10 to 14 years after Gardasil 9 vaccination for safety, immunogenicity, and effectiveness against clinical diseases related to the HPV types in the vaccine.
In the long-term extensions of clinical studies Protocols 001 and 002, effectiveness was observed in the PPE population. The PPE population consisted of individuals: – – who received all 3 vaccinations within 1 year of enrolment, without major deviations from the study protocol, who were seronegative to the relevant vaccine HPV type(s) prior to dose 1 and among women aged 16 to 26 years, PCR negative to the relevant vaccine HPV type(s) prior to dose 1 through one month postdose 3 (Month 7).
In Protocol 001 registry study, no cases of vaccine HPV types related high-grade CIN were observed through 13.6 years postdose 3 (median follow-up of 10.4 years) in women (n = 1,628) who were aged 16 to 26 years at time of vaccination with Gardasil 9. In Protocol 002 extension study, no cases of high-grade intraepithelial neoplasia or genital warts were observed through 11.0 years postdose 3 (median follow-up of 10.0 years) in girls (n = 872) and through 10.6 years postdose 3 (median follow-up of 9.9 years) in boys (n = 262) who were aged 9 to 15 years at time of vaccination with Gardasil 9.
Incidence rates of vaccine HPV types related 6-month persistent infections in girls and boys observed during the study were 52.4 and 54.6 per 10,000 person years, respectively, and within ranges of incidence rates expected in vaccinated cohorts of similar age (based on results from previous efficacy studies of Gardasil 9 and qHPV vaccine). (Ref – Gardasil 9 SPC medicines.ie)
- GARDASIL 9 Summary of Product Characteristics.
-
Hartwig S et al. Estimation of the epidemiological burden of HPV-related anogenital cancers, precancerous lesions, and genital warts in women and men in Europe: potential additional benefit of a nine-valent second generation HPV vaccine compared to first generation HPV vaccines. Papillomavirus Res 2015; 1:90–100.
a) With data from: Cervical cancer: Sanjose S et al. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study.
Lancet Oncol. 2010;11(11):1048–1056. Anal cancer: Alemany L et al. Human papillomavirus DNA prevalence and type distribution in anal carcinomas worldwide. Int J Cancer. 2015;136(1):98–107.
Vaginal cancer: Alemany L et al. Large contribution of human papillomavirus in vaginal neoplastic lesions: a worldwide study in 597 samples. Eur J Cancer. 2014;50(16):2846–2854.
Vulvar cancer: de Sanjose S et al. Worldwide human p papillomavirus genotype attribution in over 2000 cases of intraepithelial and invasive lesions of the vulva. Eur J Cancer. 2013;49(16):3450–3461
b) Method for the calculation for precancerous lesions: Based on the data from five countries (France, Denmark, Iceland, Norway, and Sweden) incidence rate ranges were estimated for CIN2+ by age groups, which were then extrapolated to the combined female population of the 32 included countries. The estimates for VIN2/3 and VaIN2/3 were extrapolated from data from Nygard study (2014) from Denmark, Iceland, and Norway from the period 2004 2006. For the estimation of AIN2/3, sex-specific, age-standardised incidence rates from the Danish Registry of Pathology were used, extrapolated to the female and the male population, respectively, of all 32 European countries. - WHO. Questions and answers about human papillomavirus (Published 2024) – https://iris.who.int/bitstream/handle/10665/376263/WHO-EURO-2024-5631-49185-73415-eng.pdf?sequence=1 (Accessed July 2025)
IE-GSL-00210 | Date of Preparation: October 2025

